Description
What is this rapid C test kit?
This rapid C test for sale is available to qualified healthcare professionals as well as business owners looking to secure a supply of rapid C tests for on-site testing.
This rapid test kit will give an easy-to-read result in approximately 10 minutes for an active C infection. This rapid test kit was also recently approved for shallow nasal collection, making sample collection much more efficient and comfortable.
What is included with this kit?
This C-19 Antigen Rapid Nasal Test Kit is sold in boxes of 25 individual test cassettes and includes the following:
(25) rapid test cassettes
Foil-pouched test device containing one test strip that
it is encased in a plastic device cassette.
(25) vials/extraction caps
The extraction vial contains 400 μl of extraction buffer solution.
(25) Anterior nasal swabs
Swabs for collecting shallow nasal samples
(1) Instructions for Use Insert
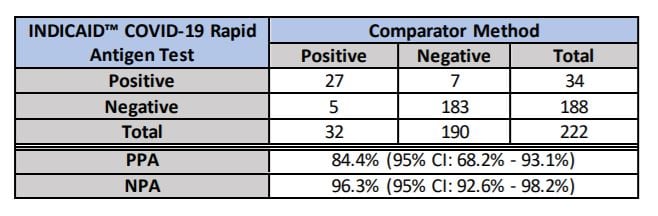
Clinical Precision Findings
Intended Use of the C-19 Rapid Antigen Nasal Test Kit
This C-19 antigen test is a lateral flow immunochromatographic assay intended for the qualitative detection of nucleocapsid protein antigen in nasopharyngeal swab specimens collected directly or collected in BD Universal Transport Media,
of people suspected of having C-19 by their health care provider within five days of symptom start.
This test is authorized for use at the point of care (POC), that is, in patient care settings operating under a CLIA Certificate of Exemption, Certificate of Compliance, o Certificate of Accreditation, The results are for the identification of the nucleocapsid protein antigen. the antigen it is usually detectable in anterior nasal swab samples during the acute phase of infection.
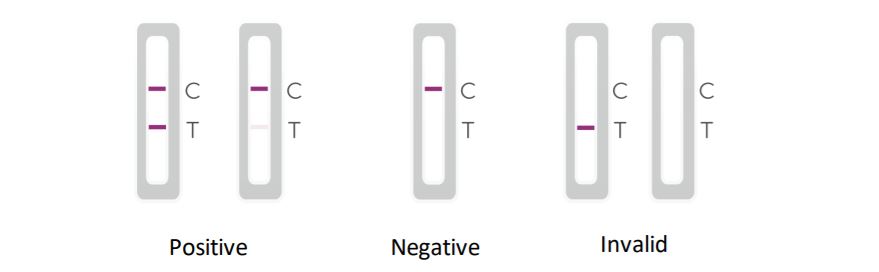
Positive results indicate the presence of viral antigens, but clinical correlation with the patient history and other diagnostic information is necessary to determine the status of the infection. Positive the results do not rule out bacterial infection or co-infection with other viruses. The agent detected it may not be the definitive cause of the disease. Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities.

Negative results are presumptive and confirmation with a molecular assay, if necessary, to patient management can be performed. Negative results do not rule out infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a
Recent patient exposures, history, and presence of consistent clinical signs and symptoms with C-19.
Indicaid C-19 Antigen is intended for use by medical or trained professionals operators who are competent in performing tests and clinical laboratory personnel trained or people trained in point-of-care settings. Indicaid C-19 Antigen is for use only under the Emergency Use Authorization of the Food and Drug Administration.
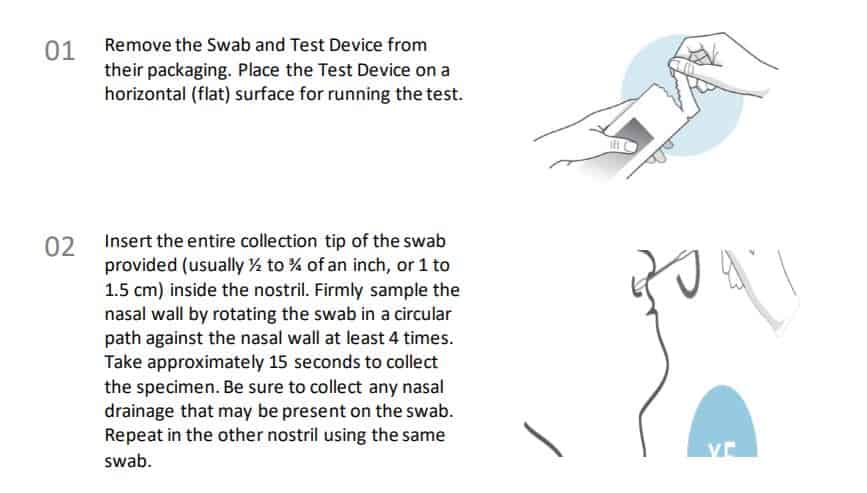
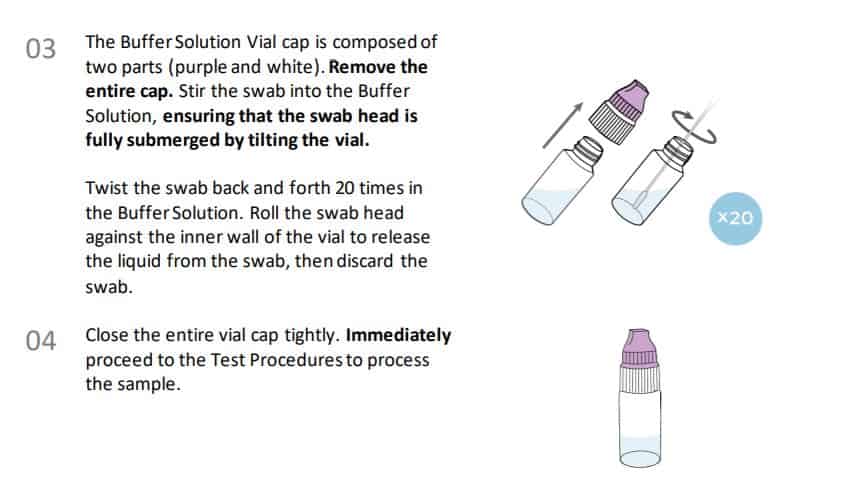
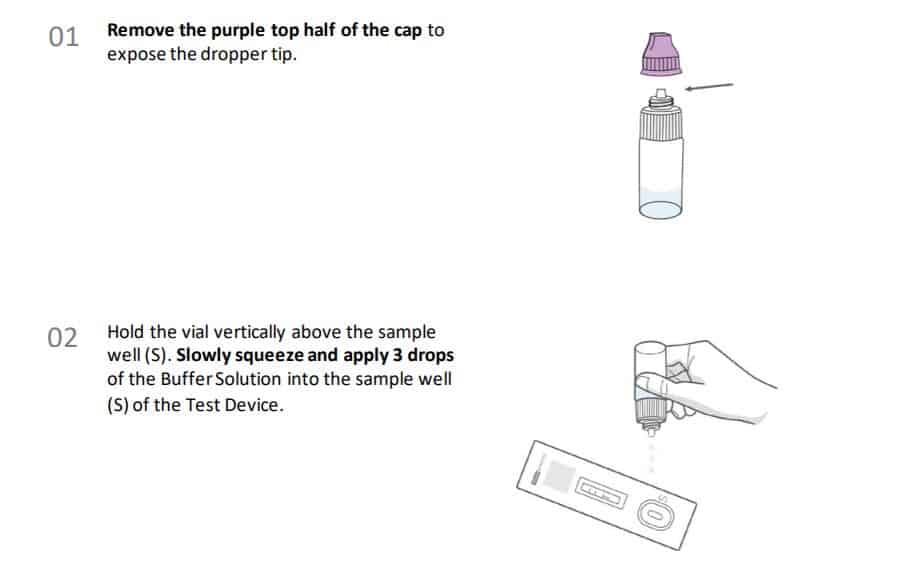
Instructions for collection and use Anterior Nasal Swab Sample Collection Procedure.
Procedural notes
• Process the test sample immediately after collection.
• Use only the supplied or recommended anterior nasal swab for specimen collection.
• Collect the sample wearing safety gloves to avoid contamination.
• Do not touch the tip (sample collection area) of the swab.
• Collect samples as soon as possible after the onset of symptoms.
Additional notes
- Allow test devices, reagents, samples, and/or controls to equilibrate to room temperature. temperature (15~30°C) before testing.
- Remove the Indicaid™ C-19 Antigen test device and extraction vial from its foil bag immediately before testing.
- The Indicaid™ C-19 Antigen Kit IS INTENDED for use only with a nasopharyngeal swab specimen or a swab in BD Universal Transport Medium.
- The Indicaid™ C-19 Antigen Kit IS NOT INTENDED to test other liquids samples such as nasal wash or aspirate samples, as results may be compromised for more than dilution.
Summary of antigen tests
C-19 antigen tests are designed to detect virus protein antigen from nasal swab samples. Antigen tests can detect the virus during the active phase of infection. The collection process is just a couple of steps, and the results are directly on site in about 10 minutes without the need for machinery. Providers can quickly and effectively screen large numbers of patients and receive immediate results to help facilitate timely treatment decisions when using a rapid antigen test kit.
Like a PCR test, rapid antigen tests can detect the presence of the virus during the acute stage of infection. Although much more efficient in providing timely results, rapid antigen tests may have a lower sensitivity rating compared to PCR tests. Any negative rapid antigen test result should be verified with a PCR test confirmation to properly diagnose and treat the case.
How To Properly Use A Rapid Antigen Test

















